What Are Neuromodulation Devices?
Reviewed by: HU Medical Review Board | Last reviewed: December 2024 | Last updated: March 2025
Anti-seizure medicines do not always control someone’s epilepsy. Sometimes the side effects of these drugs cause significant issues. Or, a combination of multiple drugs simply is not enough to achieve seizure control. If drugs and epilepsy surgery are not possible or have not helped reduce seizures sufficiently, neuromodulation devices may be an option.
Neuromodulation devices send small electrical currents to the brain. In simple terms, these electrical currents help prevent seizures by overriding the overactive electrical signals that trigger seizures.1-3
Neuromodulation devices may be prescribed for people with drug-resistant (refractory) epilepsy. This is about 1 out of 3 people with epilepsy. Neuromodulation can also be used in people who cannot get epilepsy surgery or people whose epilepsy surgery failed. It is prescribed less often for people who have trouble taking their anti-seizure drugs consistently. These devices have been in use since the 1980s.1-3
Types of neuromodulation devices
There are 3 main types of devices sold in the United States:3-6
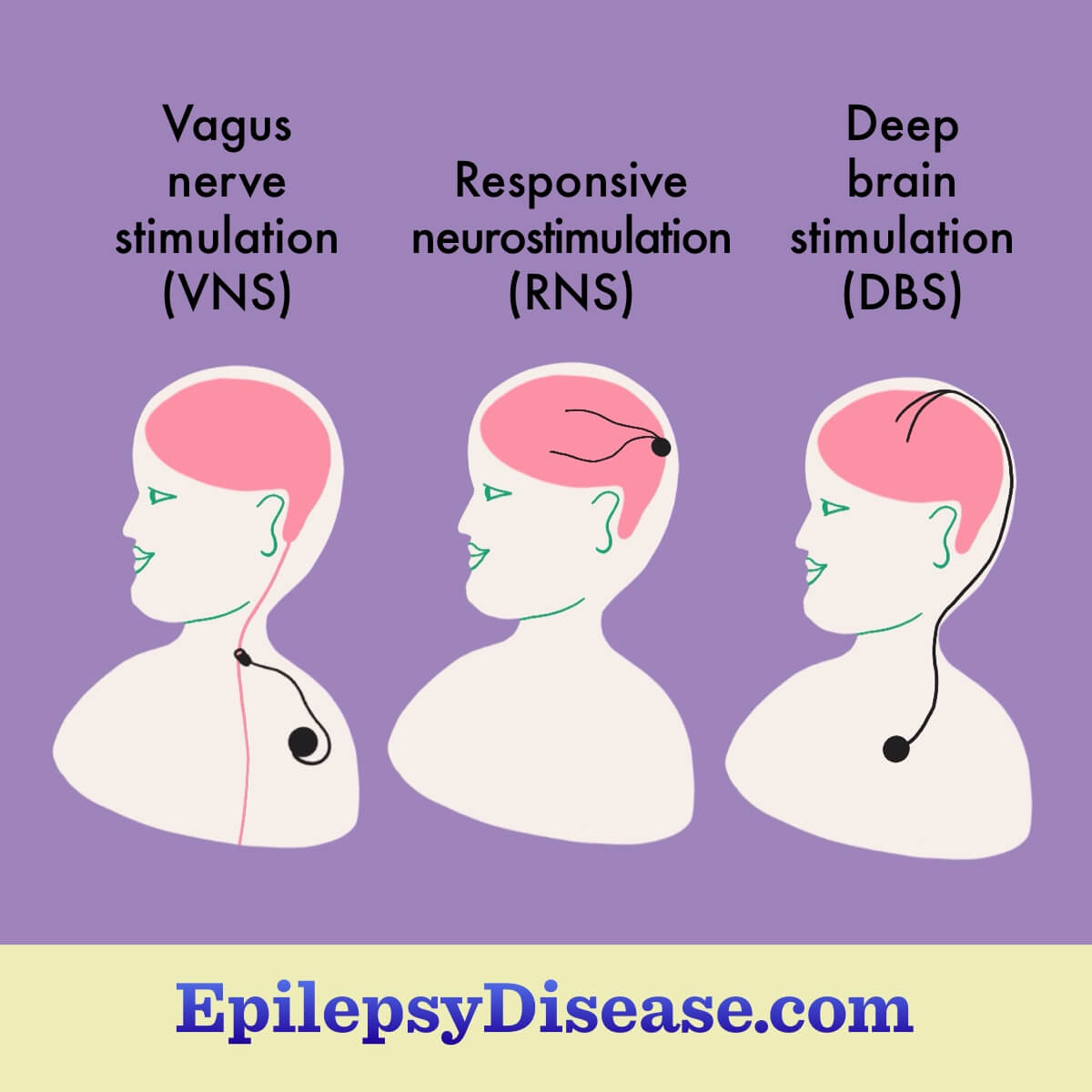
- Vagus nerve stimulation (VNS)
- Responsive neurostimulation (RNS)
- Deep brain stimulation (DBS)
Each of these devices works slightly differently and is implanted during surgery. Some are more visible, while others are easy to hide under clothing or hair. One part of the device is implanted under the skin below the collarbone or on the skull. Wires run under the skin up to the problem area of the brain. Most people do not feel the electrical pulses.3-6
Surgically implanted devices
Vagus nerve stimulation
Vagus nerve stimulators (VNS) reduce seizure activity in the brain in ways that are not fully understood but involve activating the vagus nerve. They are generally prescribed for focal onset seizures. These seizures occur in a small part of the brain and the person can remain aware. VNS devices work for some forms of generalized epilepsy too. VNS is approved in the United States for use in those ages 4 and older.2,3
A vagus nerve stimulator is battery-powered, similar to a heart pacemaker. The electrical signals it sends out are adjusted as needed to stop seizures.3
In addition to controlling seizures, a VNS may improve quality of life, mood, and daytime sleepiness. The most common side effects of VNS include:3
- Changes to the voice
- Hoarseness, throat pain, and coughing
These are not all of the possible side effects of a VNS device. It may not be an option for people with certain heart conditions or severe sleep apnea.3
Results of VNS can vary greatly. Some people have dramatically fewer seizures. Others have only moderate success in reducing the number of seizures. More research is needed to better understand which people most benefit from VNS.2,3
Responsive neurostimulation
Responsive neurostimulation (RNS) is available for people 18 years and older and those with drug-resistant epilepsy. RNS may be an option when epilepsy surgery is not possible or has not worked. RNS works by monitoring brain waves at the site of seizures and quickly detecting abnormal electrical activity that can lead to seizures. If this happens, electrical stimulation is released to prevent seizures.2,4
RNS may be used for several types of seizures:2,4
- Focal seizures caused by 1 to 2 areas in the brain
- Frequent, disabling seizures
- Focal seizures with limited awareness
- Focal to bilateral tonic-clonic seizures
RNS devices are placed outside the skull and have wires that connect to parts of the brain where seizures are thought to happen. The device cannot be seen, and its stimulations cannot be felt. Common side effects are pain around the implant site and headaches. In studies, RNS reduces seizures by 50 percent after 2 years and 65 percent after 6 years.2,7
Deep brain stimulation
Deep brain stimulation (DBS) is available for people ages 18 and older with drug-resistant epilepsy who may not be candidates for epilepsy surgery. It may be used for focal or focal-to-generalized seizures.2
A DBS device is placed by a neurosurgeon. It works by providing electrical stimulation in the brain to reduce seizure frequency or severity. A DBS is set and programmed by your doctor and can be changed as needed.2
In studies, DBS achieves a 40 percent to 56 percent reduction in seizures. Possible side effects of deep brain stimulation devices are dependent on the site of stimulation and include:2,6
- Numbness or tingling
- Muscle tightness in the face or arm
- Speech problems
- Balance issues
- Lightheadedness
- Vision problems
- Unwanted mood changes
DBS is also used for Parkinson’s disease, essential tremor, and obsessive-compulsive disorder.6
Things to know about neuromodulation devices
There are several practical issues related to these devices, such as:3,5
- MRI scans may not be possible with a neuromodulation device
- Devices that depend on batteries may lead to more frequent seizures as the battery ages
- Batteries generally last 2 to 4 years and are replaced during surgery
- These devices and the surgery they require can cost much more than anti-seizure drugs
- These devices may reduce the rate of sudden unexpected death in epilepsy (SUDEP)
There is a fourth neuromodulator device for epilepsy sold in Europe, but in the United States, it is only used for children with ADHD. It is the trigeminal nerve stimulation (TNS) device. It is still under study in the United States for use in people with epilepsy.4,8,9